
Know Everything About Product Labelling Requirement in India
Labelling products is an important aspect for manufacturers and exporters aiming to sell products within India. When a consumer buys something, they probably check the label first, that’s because labels give important information about a product. However, product labelling not only ensures compliance with regulatory standards but also directly influences
Labelling products is an important aspect for manufacturers and exporters aiming to sell products within India.
When a consumer buys something, they probably check the label first, that’s because labels give important information about a product.
However, product labelling not only ensures compliance with regulatory standards but also directly influences consumer satisfaction and trust.
So, it is important to understand that labelling requirements and packaging specifications are integral for manufacturers seeking to enter the Indian market successfully.
In this article, we are going to tell you everything about product labelling from importance to basic details related to labelling and so on.
Let’s start with understanding what product labelling is and its importance.
What is Product Labelling?
Product Labelling is creating and displaying product information on product packaging.
In simple words, on the label, a consumer can find all the information they want to know about the product before they consume it.
To make the product packaging easier for consumers, the product label must include the brand colours, the logo, the material, and the package shape.
In addition to the labelling mentioned above, the product label should include product information and a written part.
Importance of Product Labelling
In India, product labelling serves as the primary medium through which consumers obtain crucial information about a product.
Whether it’s the composition, nutritional information, manufacturing date, expiry date, or handling instructions, the label communicates vital details that influence a consumer’s purchasing decision.
Labelling Compliance for Various Industries
In India, the term labelling compliance describes the rules and regulations that regulate the information and details that a product packaging must contain.
This will guarantee the safety of consumers, the quality of the product, and the fair trade practices of the manufacturer.
Moreover, product labelling standards differ between industries depending on the nature of the product.
Below, we have listed the product labelling needs and guidelines in India for different sectors.
BIS-CRS Guidelines for Product Labelling

- Model Number
- Brand name/Trademark
- Product Ratings- Input/output (as per the registered test report)
- Name and address of manufacturing unit
- Country of Origin
- Class I, II, III or SELV Symbols (as per applicability)
- BIS Standard Mark with Indian Standard, R number and BIS Website Reference
- Symbols like Caution, do not dispose of as general/household waste, etc. To be marked on the products as per the applicability.
- The product labels may contain other declarations by Indian standards.
Labelling Requirements for BIS-ISI

So, in the BIS-ISI scheme, the marking label guidelines changed according to the product.
The marking labels guidelines are in the Indian Standard from where the reference can be taken.
The basic parameters are as follows:
- Name and type of the material
- Name of the manufacturer
- Country of origin
- Recognized trademark, if any
- Batch number/Lot number and
- Month and year of manufacture of the material.
BIS-ISI Logo
The product shall mandatorily have the BIS-ISI logo and the IS no., ‘CM/L’ and BIS website reference.
The design of the standard mark shall be identical to the facsimile given in the license.
It is also permissible to enlarge or reduce the standard mark on a photographic basis.
The BIS logo shall be easily visible, indelible, legible, and non-removable. The BIS logo is as below for generic reference.
BEE (Bureau of Energy Efficiency) Star Labelling Requirements

- Star level of Product (from 1 star to 5 stars)
- More stars, more savings/ More stars, more Light (for TFL)
- Unique series code
- Logo of the Bureau of Energy Efficiency
- Electricity Consumption/Energy Efficiency/Power Savings Guide/Fuel Saving Guide (this heading may vary from product to product)
- Name of brand / Trade name
- Model and year of manufacturing or import
- Technical details and energy consumption rating parameters (which may vary from product to product for example.. Ceiling fan power input (in watts), air delivery (in cu m/min), service value in cu m/min/Watts).
- Label period
- QR code (It is mandatory to affix the QR (Quick Response) code along with the BEE star label for Direct Cool Refrigerator (DCR) & Frost-Free Refrigerator (FFR) from January 2023, upon scanning the said QR code, all technical specifications of the model/models can be validated from BEE’s database and for other products it’s under the voluntary stage.
- General remark: Under test conditions, when tested by relevant standards. Actual electricity consumption will depend on how the appliance is being used.
- For Fixed and Variable Air Conditioners and light Commercial Air Conditioners, it is required to affix the Default Setting at the 24-degree sticker.
Also, BEE has introduced two variants of these labels i.e.:
Comparative Label

- Allows consumers to compare the efficiency of all the models of appliance/equipment to make an informed choice.
- It shows the relative energy use of an appliance/equipment compared to other models available.
Applicable to Refrigerators, air-conditioners, water heaters and washing machines.
Endorsement Label

- Defines a group of appliances/equipment as efficient, when they meet minimum energy performance standards (MEPS) specified in the respective appliance/ equipment schedule/ gazette notification.
Applicable to: Ceiling fans, tube lights, computers/laptops and televisions.
Method of Affixing the Label
The label should be affixed in such a manner that it is not possible to easily remove the label from the appliance/equipment.
The following are some of the approved methods of applying labels as prescribed in the schedule/regulation of the appliance/equipment:
- Printed self-adhesive labels, adhesive tapes, transfix labels, etc.;
- Printing on an anodized name-plate and
- Printing on appliances/equipment, like tubular fluorescent lamps and LED Lamps.
Placement: The label shall be affixed on the front/side/top of the product. The label shall also be displayed on the packaging.
TEC/MTCTE Labelling Requirements

TEC Logo
- It can be engraved/ raised/ embossed/ debossed or a printed label.
- The colour composition (RGB=0,108,156) must be maintained with no significant variation in colour in the case of the coloured label.
- Black and white labels are also permitted.
- The durability of the label shall be tested as per ISO 28219:2017.
Size of the label
- The height shall be 1/4th of the size of the brand name subject to a minimum height of 6 mm.
- If the size of the brand name is less than 6 mm due to space constraints, then the height of the TEC logo may be kept as the size of the brand name.
Label Placement
- It must be on the body of the equipment.
- In case of a removable or user-replaceable outer cover, (e.g., back cover in case of a few mobile models), a TEC certification label can be placed below the removable cover.
- TEC certificate information must be mentioned on the packaging to avoid issues at the time of import at customs.
Indication of conformity
- The technical/user manual of the product should contain information that the product conforms to the relevant Essential Requirements of TEC.
Exemption (whichever is later out of the below scenarios)
- Exempted for an initial period of 6 months with effect from the date of the TEC MTCTE phase-mandatory date.
- 6 months from the date of issue of the MTCTE certificate
FSSAI Mandatory Declaration for Packaging and Labelling

- Name of food on the pack (with tradename or description)
- List of ingredients in descending order by weight and volume
- Nutritional information
- Veg/Non-veg logo
- Specific declaration of food additives and colour/flavour
- Name and address of the manufacturer
- Net quantity
- Retail sale price (Inclusive of all taxes)
- Consumer care details
- Lot no/Code no/Batch identification
- Date of manufacturing/packaging
- Best before date and use by date
- Country of origin for imported food
- Instruction for use
- FSSAI License number with logo
- Allergen information
- Jaivik logo or Fortified logo for organic food or Fortified food
Labelling Requirements for Waste Management
Packaging and labelling are primarily the responsibility of the waste generator. The following are the labelling requirements for different waste categories:
1. E-waste Management
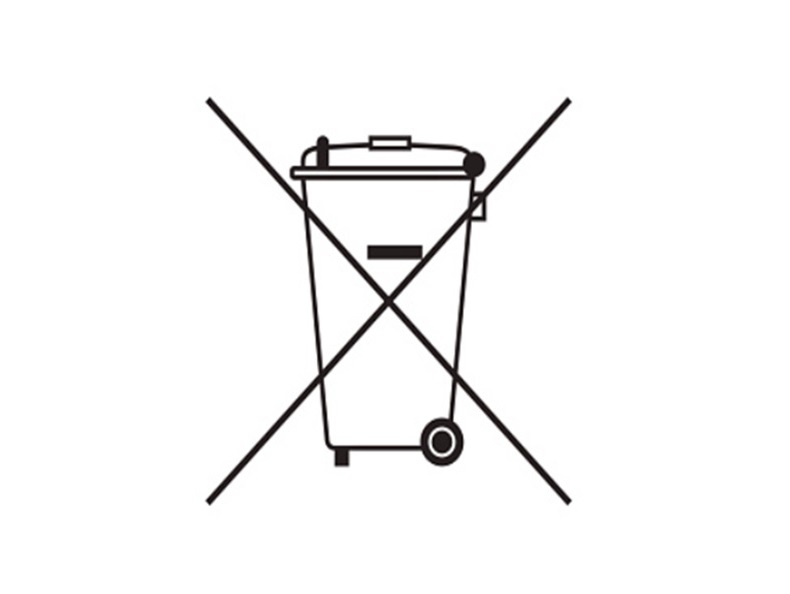
Producer shall affix a visible, legible and indelible “crossed out wheeled bin symbol” on the products or product user documentation.
2. Plastic Waste Management
The following are the labelling guidelines for plastic packaging:
- Each plastic packaging shall have the following information printed in English namely:
- Name and registration certificate number for producer importer or brand owner generated through a centralized online portal in case of:
-
- Rigid plastic packaging
- Multi-layered plastic packaging
- Compostable plastic
- Name and registration certificate number for producer, importer, or brand owner generated through centralized online portal and thickness in case of flexible plastic packaging.
- Name and certificate number issued by CPCB in case of biodegradable plastic.
- Each recycled carry bag shall bear a label or a mark “recycled” as shown below and shall conform to the Indian Standard: IS 14534: 1998 titled “Guidelines for Recycling of Plastics”.

- Also, each carry bag made from compostable plastics shall bear the label “compostable” and shall conform to the Indian Standard: IS or ISO 17088:2008 titled Specifications for “Compostable Plastics”.
3. Battery Waste Management
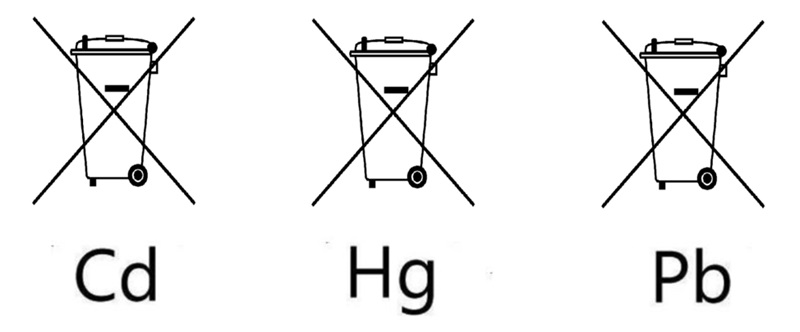
- No person shall place on the market any Battery or Battery pack unless it is marked with the “crossed out wheeled bin symbol”.
- No person shall place on the market a Battery or a button cell containing mercury, cadmium or lead unless it is marked with the respective chemical symbol “Cd”, “Hg” or “Pb”.
Legal Metrology Packaged Commodity Labelling Requirements

Let’s take a closer look at the mandatory declarations that must be included on product labels/packaging.
- Generic name of the product
- Country of origin
- Size in cm/m where needed
- Month and year of manufacturing
- Customer care number and e-mail.
- Maximum retail price (in Rupees)/ unit sale price
- Manufacturer/importer/packer name and complete address with full PIN code
- Quantity/Volume/Net/Weight: In number, unit or any other word representing quantity or in matric notation i.e. kg, g, l, ml etc.
Mandatory Labelling Requirements for Cosmetic

The following information needs to appear on the label on the container as well as any external packaging.
- Name of the cosmetic
- Name of the legal manufacturer
- Use before date/date of expiry.
- The full address of the manufacturing site of the cosmetic.
- A list of ingredients with a concentration of greater than one per cent should be listed in ascending order at the time of addition.
Those with a concentration less than or equal to one per cent, in any order, must precede this list with the words “INGREDIENTS.”
Inner or Outer Labelling Requirements
The following information needs to appear on either the inner or outer label.
- Distinctive batch/lot code* [preceded by “Batch No.”, “B. No”, “Lot No.” or “Lot”]
- Manufacturing License Number* [preceded by “M”, “M.L. No.”, or “Mfg. Lic. No.”]
It is advisable for both the outer and inner labels should contain the license number and the batch code.
Most regulators check the outside label for compliance, however, most consumers throw away the secondary package when they unpack it.
Information to be Highlighted only on the Outer Label
The following information only needs to appear on the outer label:
- Net contents (weight for solids, fluid measure for liquids, and either for semi-solids)
- Number of items, if more than one.
Information to be Highlighted only on Inner Label
The following should appear if a cosmetic has any hazards:
- Directions on how to use
- Any warning, caution or special directions
- Names and quantity of hazardous and poisonous ingredients
If this is not the case, only declarations that must be made on both the inside and the outside of the container must be indicated.
Medical Devices Labelling Checkpoints
Medical device labels and instructions must meet the requirements of the Medical Device Regulations (MDR) 2017.
Labels and instructions of use should meet the requirements of additional ISO Standards.
The MDR has over 20+ checkpoints for manufacturers or importers to meet. So, here are some of the checkpoints related to medical device labelling:
Basic Labelling Requirements for Medical Devices
- CDSCO-required format should be used for the full name.
- Medical device information, including identification and use, must be included.
- Clear name of manufacturer or importer along with manufacturing premise address.
- All the warning signs and precautions should be mentioned for the medical device user.
- The correct measurements for weight, volume, unit count, and number of devices in the package shall be indicated in the appropriate format.
It is mandatory to use the metric system when expressing any figures.
- In the case of sterile devices, the expiration date should be accompanied by the year and month the device was manufactured.
- In the case of devices made from non-sterile materials such as stainless steel, titanium, etc., it is not necessary to include the expiration date.
- The shelf life of the product should also be included on the label along with the expiration date.
- A clear indication of whether medical devices contain biological or medical components.
- After the words ‘Lot No’ “Lot” “Batch No” or “B.No”, a unique batch number, or lot number, should be indicated.
- If applicable, the statement should also include the specific storage and handling conditions for the medical device.
- All medical devices that are sold as sterile must clearly state on the label that they are sterile and what type of sterilization they use.
- It is also important to mention whether the device is designed for single-use or multi-use.
- To provide a medical professional with a free sample of a device, the label must read ‘Physician’s Sample – not to be sold’.
- The manufacturing license number must appear on the label in the form of “Manufacturing License Number” or “Manufacturing.Lic.No” or by using “M.L.”.
Labelling Requirements for Imported Medical Devices
-
- Importing license number
- Importer’s name with their address
- Address of their actual manufacturing site
- Date of Manufacturing
- On the label of imported medical devices, include the international and national symbols of certification, such as the Bureau of Indian Standards (BIS), International Organization for Standardisation (ISO), etc.
Penalties for Inaccurate Labelling

Inaccurate labelling of products can be misleading and can result in loss of consumer interest and trust.
In India, several laws and penalties can be imposed for non-compliance with labelling regulations, ranging from fines to imprisonment.
For example, the Advertising Council of India an independent organization regularly issues legal notices to advertisers for making exaggerated claims about their products like “get fair skin in 7 days.”
As a result, brand owners must recall their products as soon as possible and are subject to heavy fines.
Vincular – Your Compliance Partner

Vincular is India’s best regulatory compliance company providing certification, testing and consultation services since 2012.
Moreover, our knowledgeable team of experts with 300+ years of collective experience serves diverse industries ranging from Consumer Products, Electronics, Telecom, Medical and Cosmetics to Automotive, and more.
You can be confident that you are in good hands when you choose us as your compliance partner.
Note – Subscribe to our WhatsApp Channel to get the latest notifications and updates and stay ahead of the competition.
Conclusion
Indian product labels serve as a bridge between consumers and products by communicating essential information transparently.
Labelling compliance varies across industries, from composition details to safety measures, demanding adherence to specific guidelines.
Neglecting accurate labelling not only impacts consumer trust but also invites legal repercussions.
Therefore, for manufacturers and exporters eyeing success in the Indian market, meticulous attention to labelling requirements remains paramount.