Medical Devices Certification
While importing any medical device under Class A, Class B, Class C & Class D requires the certification from the Indian Government

Medical Certification
Medical Devices have always been an object of concern and regulation by the Indian Government. With advancing medical technology and the exponentially rising need of medical devices in the country, new reforms and rules have come into effect. Medical Devices Rules’2017 (MDR) are governed by respective central and state regulatory authorities. Regulatory bodies classify medical devices based upon the associated risk and have defined relatively easy procedures for license applications. Similarly, rules and guidelines have been promulgated by the government for the import of medical devices. Whereby, all one need is to have an Authorized Indian Representative (AIR) to apply for the license. With the reformative intervention and efforts of the government such as the SUGAM portal, obtaining medical device licenses is now an expedite procedure For all the medical devices along with in Vitro medical devices, being imported or manufactured, requires the permission from CDSCO. Here, the approval is given by CDSCO’s State Licensing Authority (SLA) or the Central Licensing Authority (CLA).

Notified devices under the regulation
| Device Name | Device Name |
|---|---|
| 1-Disposable Hypodermic Syringes | 19- Condoms |
| 2-Disposable Hypodermic Needles | 20- Tubal Rings |
| 3-Disposable Perfusion Sets | 21- Surgical Dressing |
| 4-In vitro Diagonostic for HIV, HbsAg & HCV | 22- Umbilical Tapes |
| 5-Cardiac Stents | 23- Blood/ Blood Component Bags |
| 6-Drug Eluting Stents | 24- Disinfectant. |
| 7-Catheters | 25- Nebulizer |
| 8-Intra Ocular Lenses | 26- Blood pressure monitoring device |
| 9-I.V. Cannulae | 27- Digital Thermometer |
| 10-Bone Cements | 28-Glucometer |
| 11-Heart Valves | 29- All Implantable Medical Device |
| 12-Scalp Vein Set | 30- MRI Equipment |
| 13-Orthopedic Implants | 31- CT Scan Equipment |
| 14-Internal Prosthetic Replacements | 32-Dialysis Machine |
| 15-Ablation Devices | 33- PET Equipment |
| 16- Blood Grouping Sera and Substances for In Vitro Diagnosis | 34- X Ray Machine |
| 17- Ligatures, Sutures and Staplers | 35-Defibrillator |
| 18- Intra Uterine Devices (Cu -T) | 36-Bone Merrow Cell Seperator |
| 37-Ultrasound Equipment |
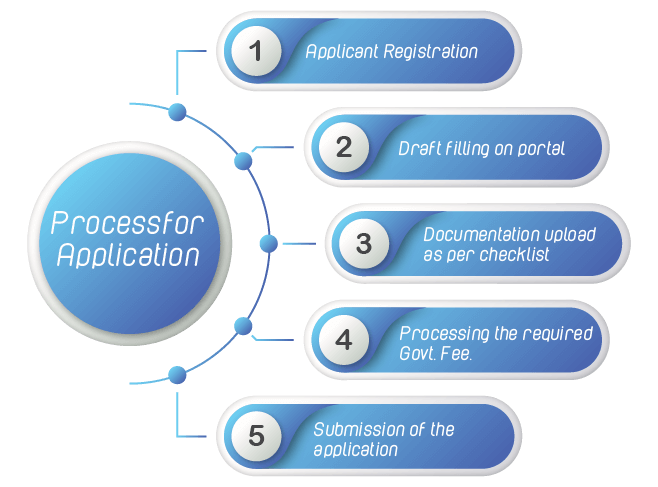
Application Process
Risk Based Classification

Medical devices have been classified into A, B, C and D categories where the risk factor involved increases from A to D. Low-risk devices include equipment like thermometers whereas high-risk devices include pacemakers, heart valves and others. The devices are further classified as surgical or non-surgical devices based upon their invasiveness. License for class A devices is easy to obtain as compared to class D devices.
Certificate Type & the applicable authorities
| Device Class Activity | Class A | Class B | Class C | Class D |
|---|---|---|---|---|
| Import | CLA | CLA | CLA | CLA |
| Manufacture | SLA | SLA | CLA | CLA |
| Permission to Conduct Clinical Investigation | Permission from CLA | |||
| QMS Verification | Notified Body registered with CLA | Notified Body registered with CLA | CLA | CLA |
Applicable Licenses
| SN | Forms | Application Type |
|---|---|---|
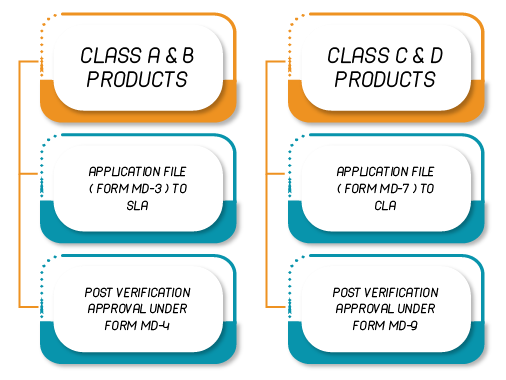
| 1 | Form MD-3 | Application for Grant of License to Manufacture for Sale and Distribution of Class A or Class B medical device |
| 2 | Form MD-4 | Application for Grant of Loan License to Manufacture for Sale or for Distribution of Class A or Class B medical device |
| 3 | Form MD-5 | License to Manufacture for Sale or for Distribution of Class A or Class B Medical Device |
| 4 | Form MD-6 | Loan License to Manufacture for Sale or for Distribution of Class A or Class B medical device |
| 5 | Form MD-7 | Application for Grant of License to Manufacture for Sale or for Distribution of Class C or Class D |
| 6 | Form MD-8 | Application for Grant of Loan License to Manufacture for Sale or for Distribution of Class C or Class D |
| 7 | Form MD-9 | License to Manufacture for Sale or for Distribution of Class C or Class D |
| 8 | Form MD-10 | Loan License to Manufacture for Sale or for Distribution of Class C or Class D medical device |
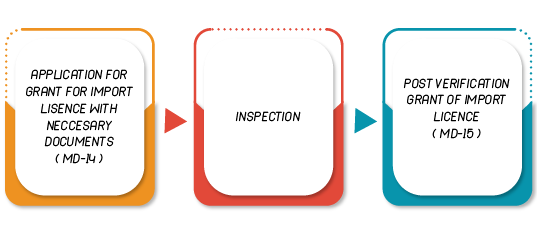
| 9 | Form MD-14 | Application for issue of import licence to import medical device |
| 10 | Form MD-15 | Licence to Import Medical Device |
| 11 | Form MD-41 | Application for Grant of Registration certificate to sell, stock, exhibit or offer for sale. |
| 12 | Form MD-42 | Registration Certificate to sell, stock, exhibit or offer for sale. |
Classification of Applicant & required license
Importer
Indian Manufacturer
Important Points
If a Manufacturer or Authorized agent, considers that a medical device which has been imported, manufactured, sold or distributed , is likely to be unsafe, such manufacturer or authorized agent shall immediately initiate procedure to withdraw the medical device in question from the market and immediate inform the competent authority.
The Medical device shall be registered with the Central licensing authority through an identified Online portal established by the Central Drugs standard Control Organization.
Applicant’s Registration Number will be generated by CDSCO, Manufacturer / Importer shall mention the registration Number on the label of the Medical Device.
The Medical device shall be registered with the Central licensing authority through an identified Online portal established by the Central Drugs standard Control Organization.
Applicant’s Registration Number will be generated by CDSCO, Manufacturer / Importer shall mention the registration Number on the label of the Medical Device.